Expanding production capacities for vaccines is an important instrument to prevent and fight global pandemics. New approaches aimed toward finding solutions for local vaccine production are being provided by BioNTech through modular containers called “BioNTainer”. With this invention – the name being a cross between container and the company name BioNTech – COVID-19 vaccines will be produced on the African continent in the future. A summit on this subject took place on 16 February 2022 in Marburg. The event to mark the official launch of BioNTech’s cooperation in vaccine production with several African countries was attended by high-ranking officials: the Presidents of Senegal, Ghana and Rwanda as well as the German Federal Minister for Economic Cooperation and Development, Svenja Schulze, representatives from the World Health Organization (WHO), the Africa Centers for Disease Control and Prevention (Africa CDC), the African Union Development Agency NEPAD (AUDA-NEPAD) and the European Investment Bank. The President of the EU Commission, Ursula von der Leyen, and Chancellor Olaf Scholz also attended the event virtually. PTB was represented at the event by the expert Dr. Christina Förg-Wimmer, who took part while she was accompanying the Ghanaian vaccine commission on a delegation trip.

Starting in 2023, vaccine production in the BioNTainer is to begin in some African countries. The aim of the African Union is to produce 60 % of the vaccines required by Africa on the continent by the year 2040. It is intended that the first modular production units will already be shipped in mid-2022. BioNTech is planning to send containers to Rwanda and Senegal first; negotiations with South Africa are taking place. This process is to be carried out in close cooperation with the African Union and the local authorities.

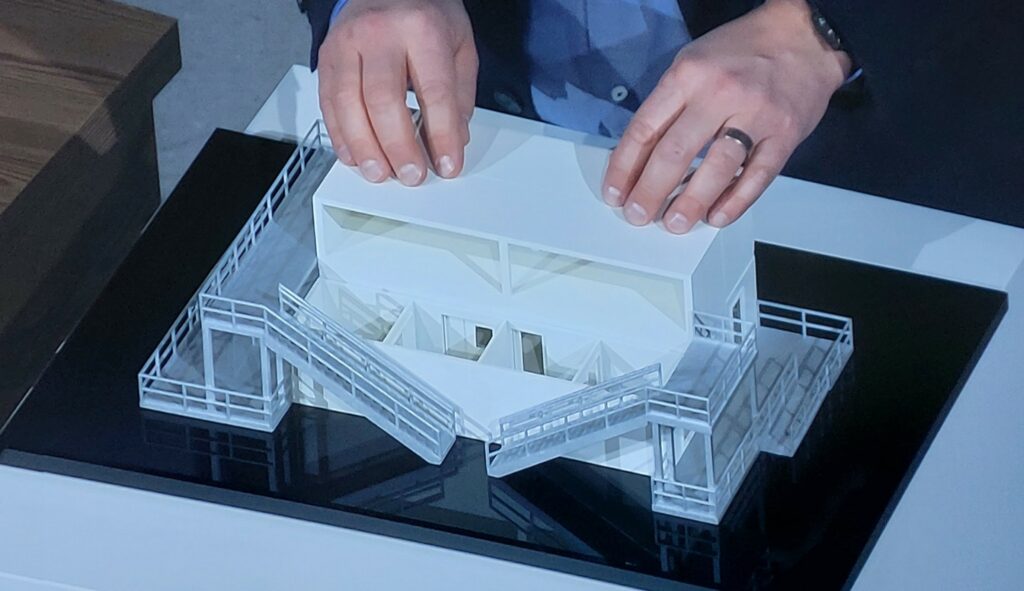
During the event in Marburg, BioNTech’s founders Prof. Ugur Sahin and Prof. Özlem Türeci explained the exact production process and how BioNTainer are set up on a model. Quality in the production process is always of the highest importance for this. In modular systems, all of the working steps can be carried out centrally and thus have the advantage of high-speed implementation. The company itself is responsible for setting the system up. The necessary infrastructure, however, is provided by the local authorities and governments. Furthermore, Ghana will support the process by providing capacities in terms of filling vials and packaging (fill & finish). The aspect of sustainability is also at the focus in the planning process. These novel approaches in vaccine production offer flexibility and have the potential to be applied to other diseases and processes in addition to COVID-19.
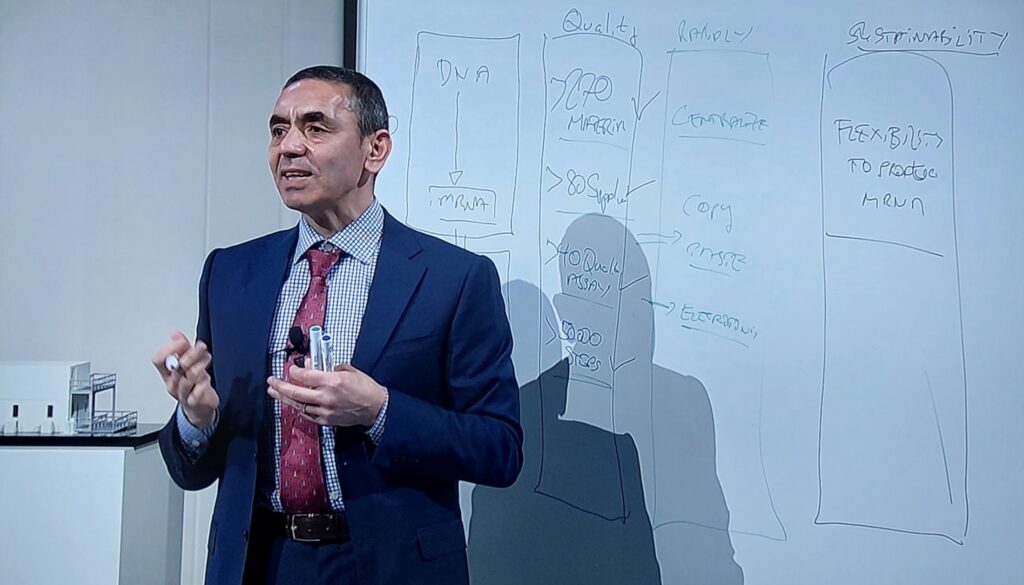
Financed by the Federal Ministry for Economic Cooperation and Development (BMZ), PTB is supporting, among other topics, the development of quality infrastructure in the pharmaceutical sector as well as quality assurance services for the local production of medications and vaccines within the scope of the Upgrading of Quality Infrastructure in Africa project. The project focuses on strengthening the regulatory framework and the quality assurance of pharmaceutical products and vaccines in Africa. The focus here lies on Ghana, Rwanda, Senegal and South Africa.
At present, PTB is strengthening the capacities of its partner countries in good manufacturing practices (GMP), especially for quality-assured sterile production which must be followed within the scope of vaccine production. In this process, the quality management systems for testing laboratories and the regulation capacities of the local authorities will be strengthened. In this context, PTB is cooperating with the Paul Ehrlich Institute, the regulatory authority for vaccines in Germany.
Photos © Christina Förg-Wimmer








